Revolutionary Technology
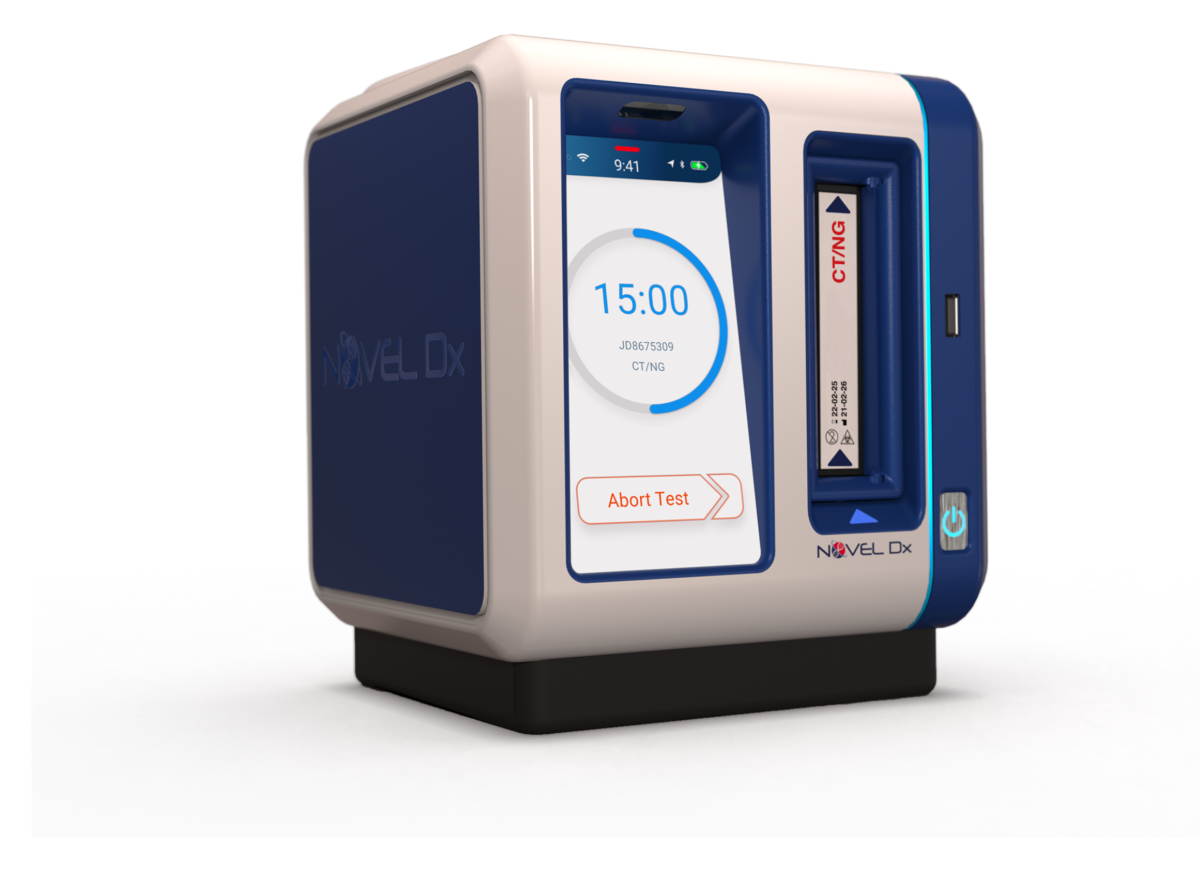
Novel Microdevices’ rapid point-of-care diagnostic is designed for portability, weighing only four pounds. It is battery-powered, making it particularly suited to low resource settings and low-and middle-income countries (LMICs).
The molecular testing platform implements polymerase chain reaction (PCR) technology on a disposable cartridge. The patient sample is added to the cartridge, which is then inserted into the Novel Dx system, which analyzes the genetic sequence of cells to identify disease and the presence of genetic mutations known to make bacteria resistant to antibiotics.
How it Works
Lab Quality and Clinically-Actionable Results in Minutes
The Novel Dx device detects the genetic material of pathogens using Nucleic Acid Amplification Techniques (NAAT). These are the same methods utilized by clinical reference laboratories. Novel Dx delivers lab-quality results in under 15 minutes so patients are tested, diagnosed, and treated in the same visit.

The Novel Dx System and Diagnostic Assays are in development and not available for sale
Product Features
ACCURATE
Equivalent to lab test
Antibiotic stewardship = savings
SENSITIVE
ULTRAPORTABLE
Weighing <4 lbs; Battery powered
Easy to use
Automated, designed for CLIA waiver
MULTIPLEXED
Rapid
Results displayed in ~15 minutes
AFFORDABLE
Multiple sample types
CONNECTED
Synced to Novel Dx Cloud

Stackable
stackable design for increased throughput

Results in less than 15 minutes
Novel Microdevices provides fast, simple and accurate results in less than half an hour instead of days.